What the FDA's Restriction of Avandia Means for Diabetes Patients
http://healthland.time.com/2010/09/2...for-diabetics/
Now that the Food and Drug Administration has decided to strictly limit the use of the diabetes drug Avandia, what does that mean for patients?
According to the FDA's statement, the agency is putting the medication, also known as rosiglitazone, on restricted access. In order to prescribe the drug, physicians will now have to be part of a registry that certifies they are familiar with the heart risks associated with Avandia, and that they are prescribing it only because their patients have exhausted all other medications to help control their blood sugar. Patients will have to sign off on the heart dangers as well. Most physicians will likely be contacting their patients on Avandia in coming days to discuss the new regulations and the option of switching to another medication.
The restrictions, and the fact that there is a similar drug that has not been associated with the same degree of heart risks, may be the death knell for the once popular medication, which grossed nearly $3 billion worldwide during its peak of popularity in the early 2000s. “Just the paperwork requirement is too daunting to put people on this drug,” says Dr. Steven Nissen, a cardiologist at Cleveland Clinic who first documented the increased heart attack rate associated with the drug in 2007. “If all you have to do is pull out a prescription pad for an alternative drug, what do you think the doctor is going to do? I think the drug is effectively gone.”
It is indeed gone from the European Union nations, whose regulatory body reviewed similar data and decided to take the further step of suspending Avandia.
Why the different actions? The European Medicines Agency determined that the heart risks of Avandia did not justify its benefit in lowering blood sugar, and given that alternatives with safer profiles are available, specifically pioglitazone, or Actos, which belongs to the same class of medications as Avandia, they decided that diabetic patients would not be harmed by removing the drug.
The FDA's Dr. Janet Woodcock, director of the Center for Drug Evaluation and Research, on the other hand, noted in the agency's statement that “the cardiovascular safety profile of rosiglitazone is still an open question because there are conflicting data on the existence and magnitude of the risk.”
At issue is a company-sponsored study that was the basis of discussions in the drug's safety review after it was approved in 1999. Some experts, including Nissen and even FDA scientists, maintain that the trial was flawed and minimized the heart risks. Others are not so sure, noting that often, diabetes patients who are put on new medications appear to fare worse in the short term but then stabilize and show stronger benefits in controlling blood sugar down the road. “There were a lot of questions raised about how to interpret the data, and there was a tremendous amount of uncertainty,” says Dr. Allison Goldfine, head of the section on clinical research at Joslin Diabetes Center and a member of the panel that voted to keep Avandia on the market. Goldfine voted to keep the drug but with the added restrictions to its use.
Even Goldfine acknowledges, however, that the FDA decision will make prescribing Avandia difficult. Sales of the medication began dipping after Nissen's paper was published and the FDA issued its first warning, advising patients with a history of heart disease from taking the drug. There will be “very few” patients, she says, who will fulfill the criteria necessary to take the medication, since at the moment, there is no known medical reason that people with Type 2 diabetes would not be able to use any of the other glucose-controlling drugs currently on the market. Says Nissen, “I find it hard to imagine that even a single patient can't take any of the other diabetes drugs. Nobody should essentially get this drug.”
Please visit our sponsors
Results 1 to 1 of 1
-
24-09-2010, 05:19 AM #1Investor

- Join Date
- Sep 2010
- Posts
- 349
- Feedback Score
- 0
- Thanks
- 190
- Thanked 233 Times in 149 Posts
 What the FDA's Restriction of Avandia Means for Diabetes Patients
What the FDA's Restriction of Avandia Means for Diabetes Patients
-
Sponsored Links
-
Sponsored Links
Thread Information
Users Browsing this Thread
There are currently 1 users browsing this thread. (0 members and 1 guests)
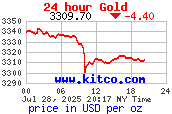
24 Hour Gold
Advertising
- Over 20.000 UNIQUE Daily!
- Get Maximum Exposure For Your Site!
- Get QUALITY Converting Traffic!
- Advertise Here Today!
Out Of Billions Of Website's Online.
Members Are Online From.
- Get Maximum Exposure For Your Site!
- Get QUALITY Converting Traffic!
- Advertise Here Today!
Out Of Billions Of Website's Online.
Members Are Online From.






 LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks



 Reply With Quote
Reply With Quote